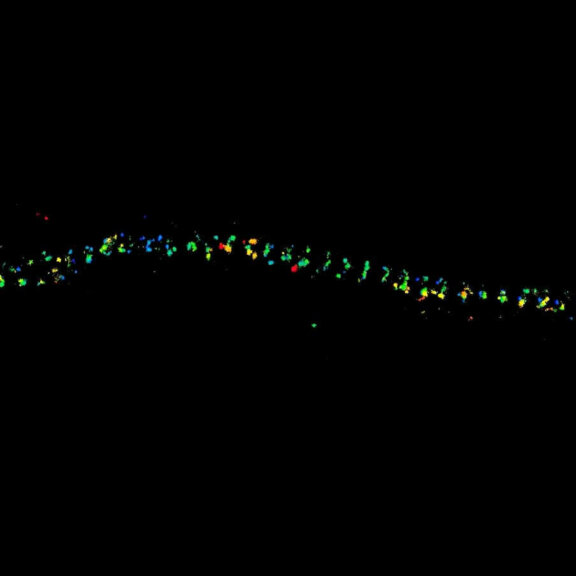
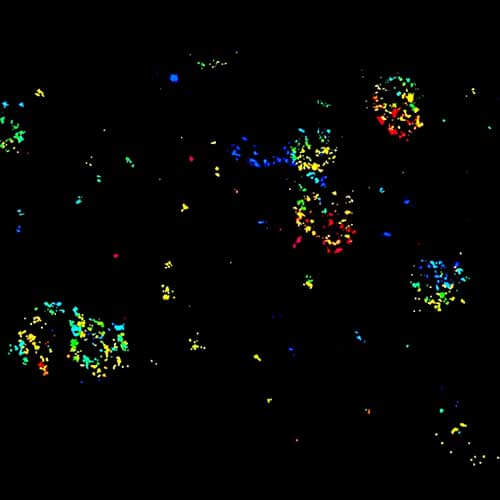
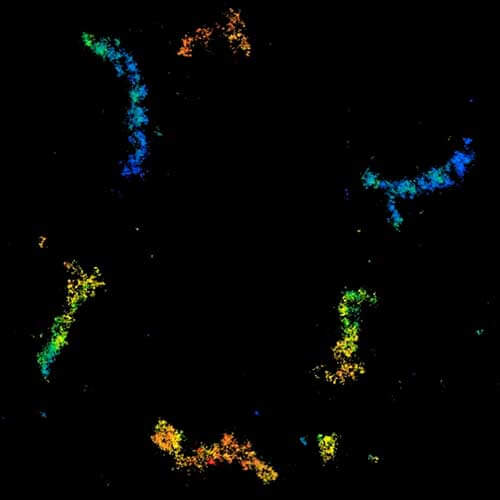
MINFLUX 3D
The MINFLUX platform offers an unprecedented array of imaging possibilities and allows you to resolve structures as small as a molecule, along all three dimensions.
This unmatched resolution capability combined with unprecedented speeds reveals sample details never seen before. The MINFLUX is the world’s most powerful fluorescence microscope.
unrivaled resolution and speed
100x sharper
than a confocal microscope
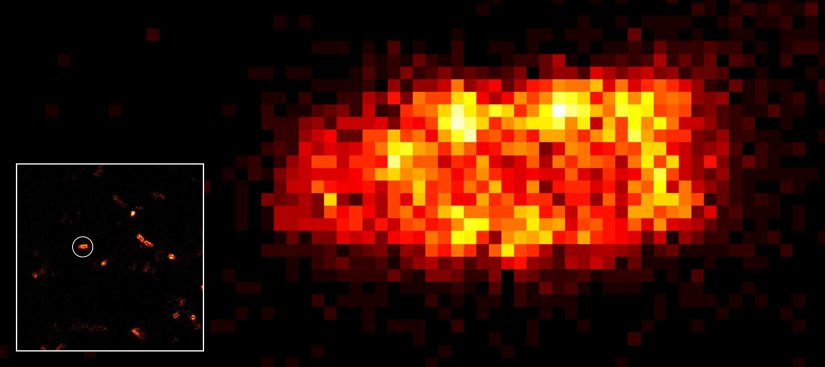
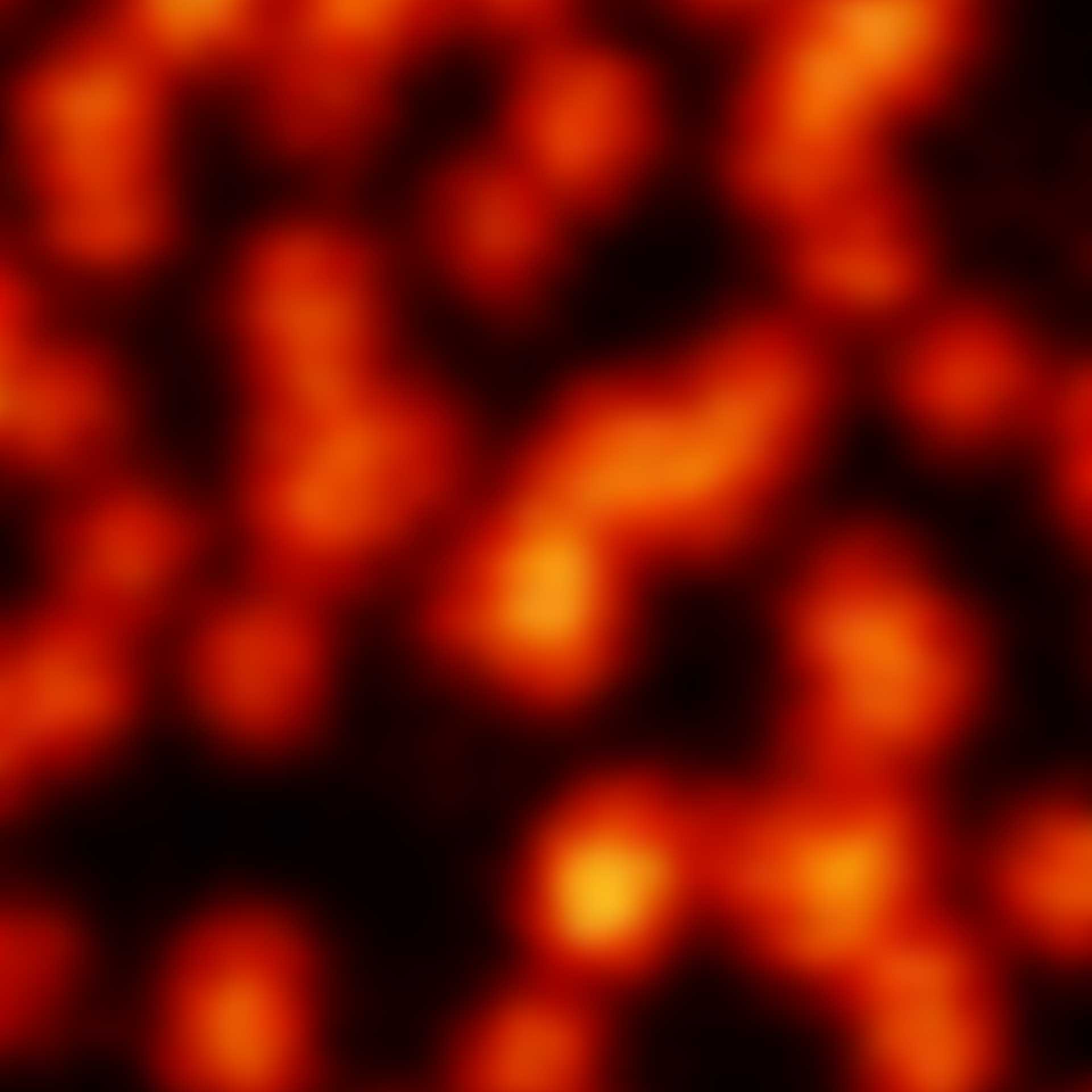
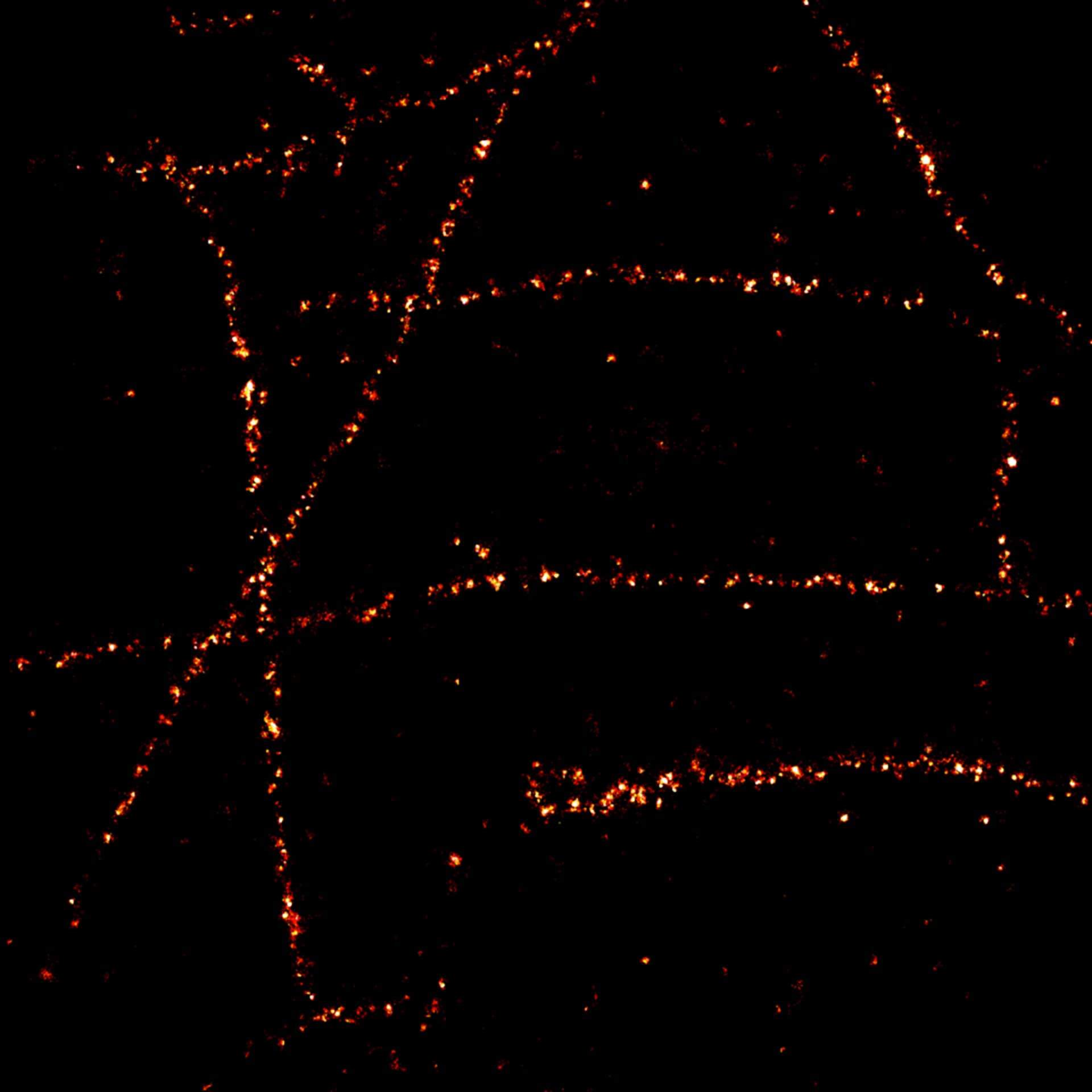
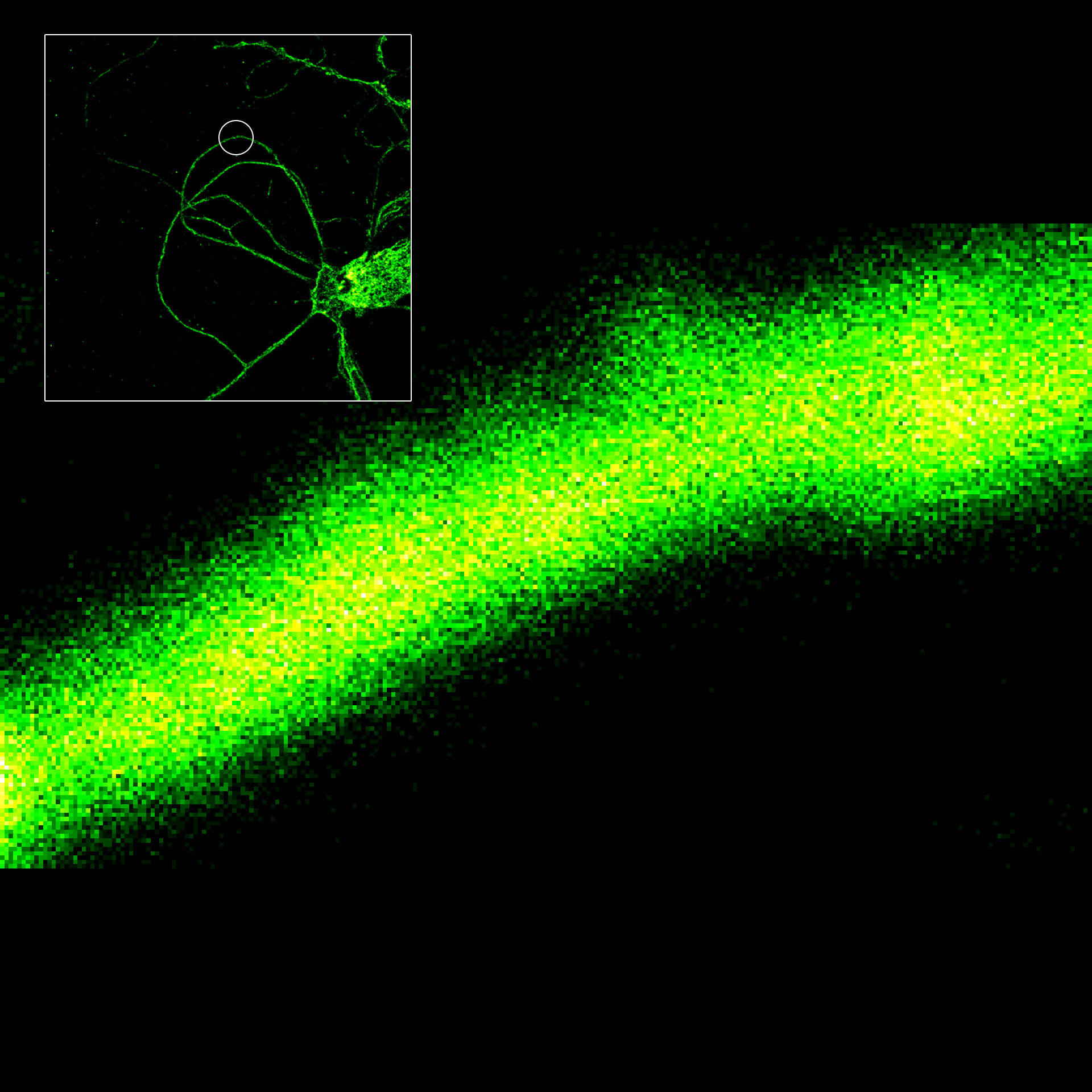
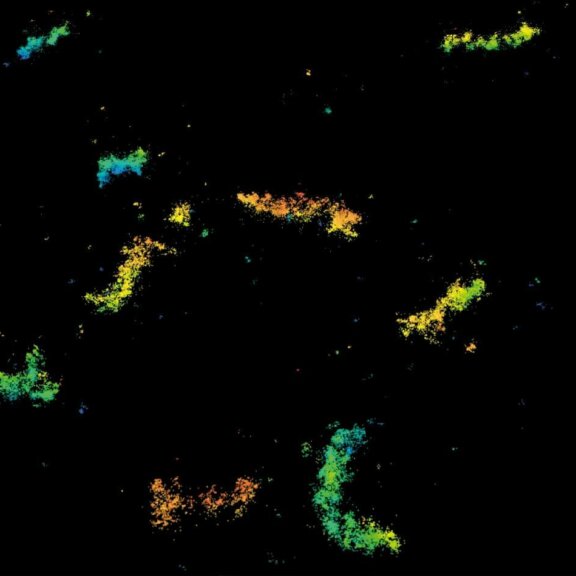
Although impactful, previous superresolution methods have failed to achieve resolutions on the length scales of the fluorescent molecules. Harnessing an entirely new and revolutionary localization principle, MINFLUX has finally accomplished this feat. Expect a resolution leap of 10-fold compared to other superresolution methods and 100-fold compared to confocal fluorescence imaging.
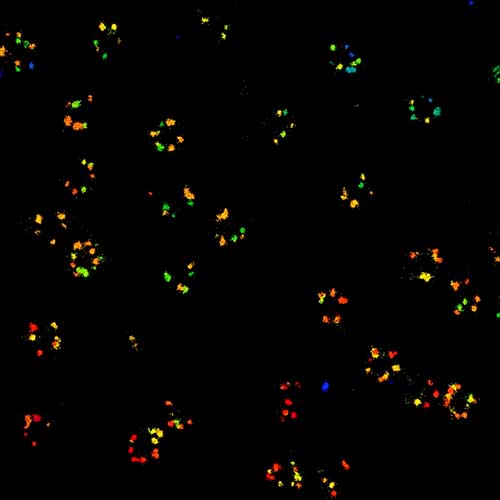
MINFLUX is the most precise and most photon-efficient way of localizing fluorescent molecules. With its new MINFLUX system, abberior has produced the first commercial fluorescence microscope that can achieve localization precisions of 1-3 nm (3D) in biological samples on large fields of view (10 x 15 μm). MINFLUX imaging means getting the maximal localization information out of your sample.
100x faster tracking
than a camera
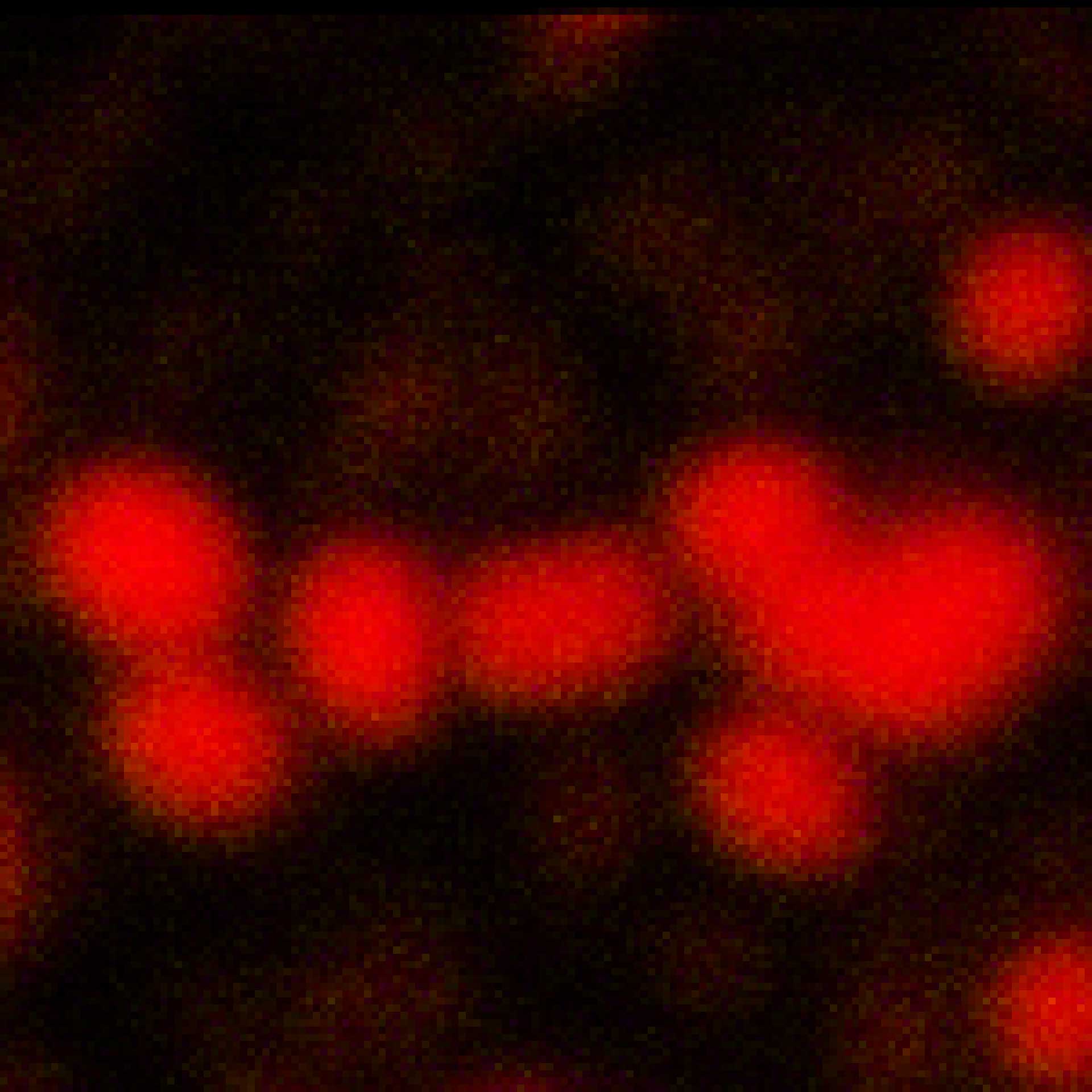
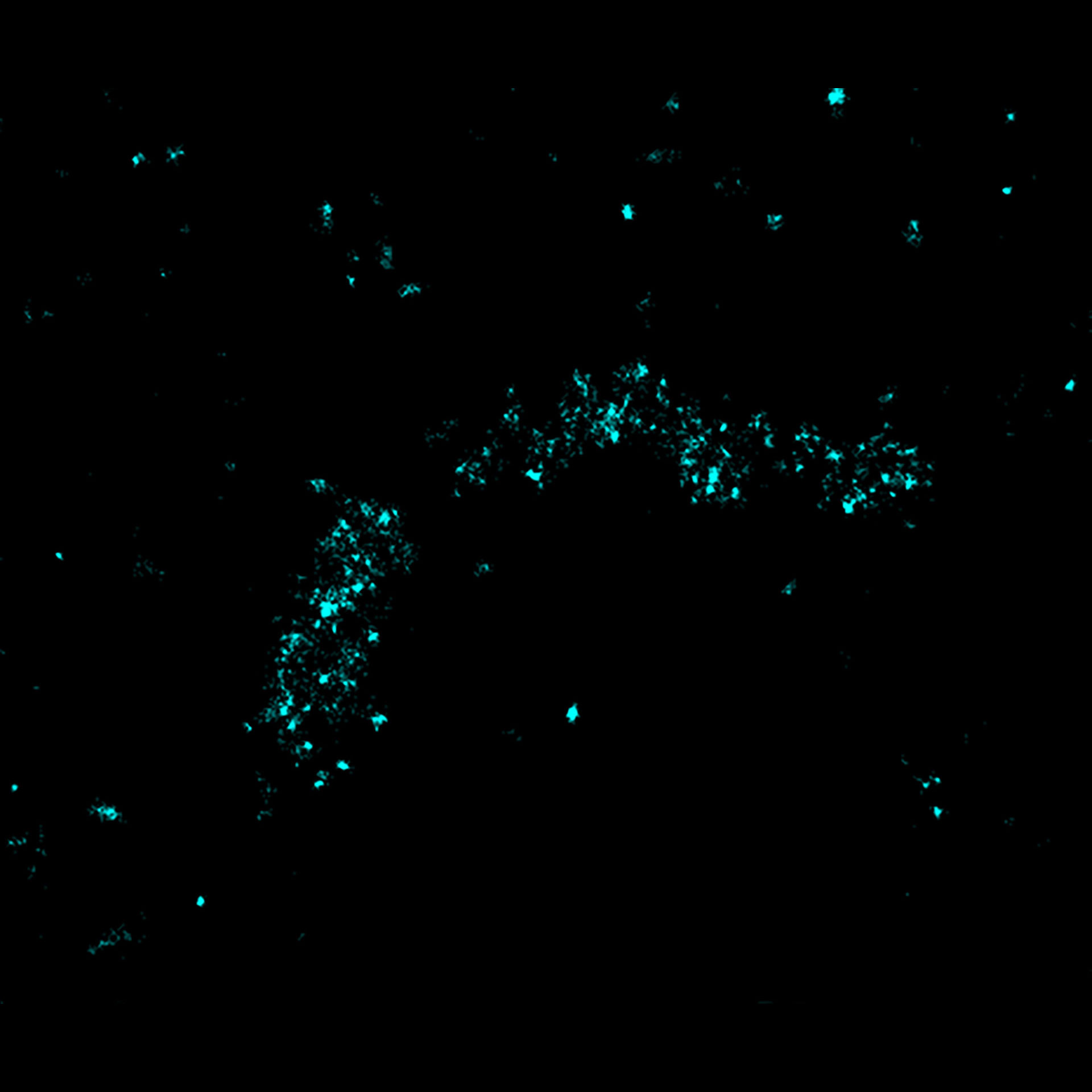
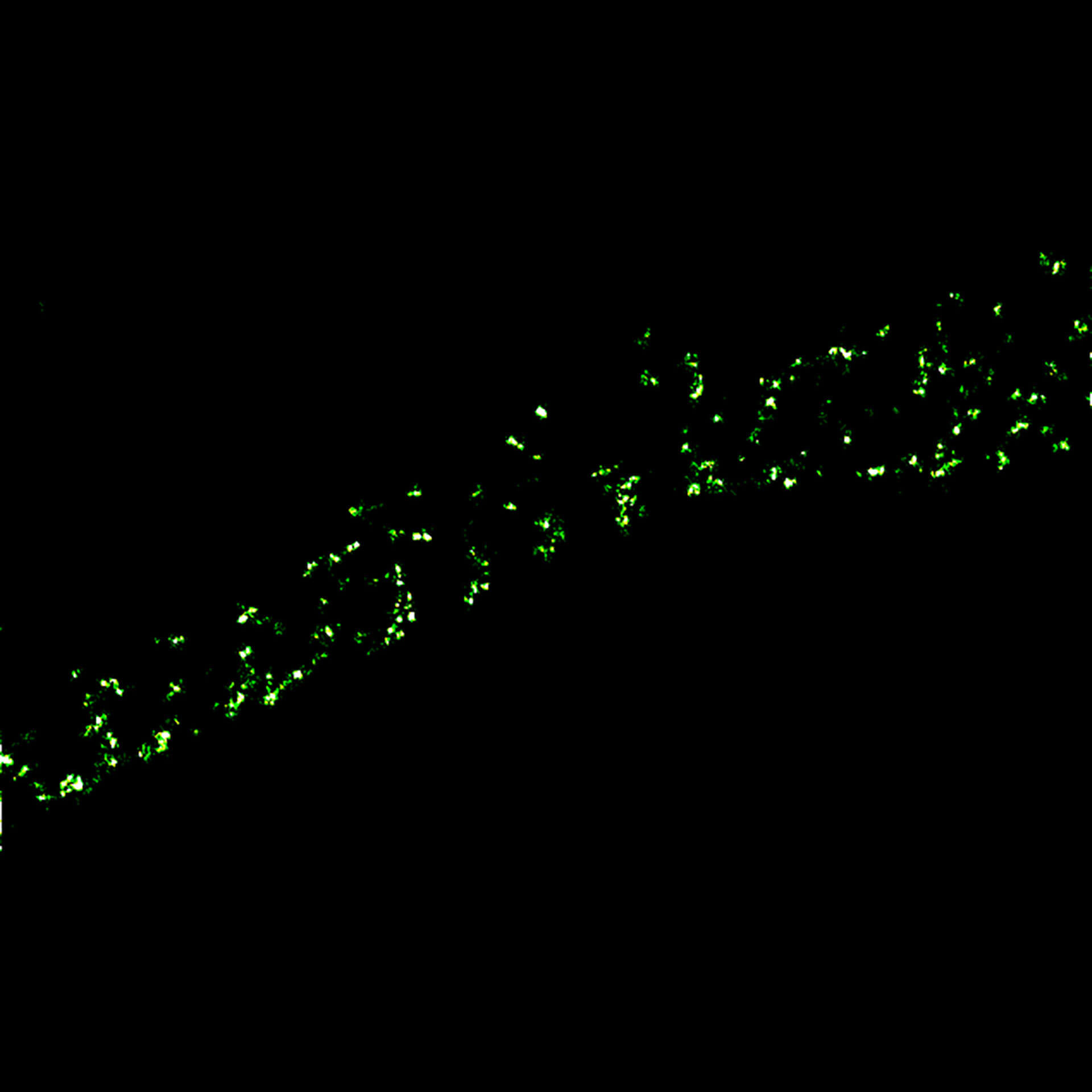
MINFLUX tracks molecular movements at frequencies up to 10 kHz, resolving molecular motion every 100 μs. That’s 100 times faster than conventional camera-based methods. Of course, tracking works in all three dimensions, i.e. along x, y, and z. Due to the low number of photons required for each localization, single molecules can be monitored with unprecedented spatio-temporal resolution (e.g. 28.000 localizations each at 20 nm resolution).
MINFLUX is the fastest way to localize fluorescent molecules. With its revolutionary MINFLUX microscope, abberior is raising the bar for molecular tracking with world-record temporal resolution, opening new doors for life-scientists across all disciplines.
Choose between MINFLUX molecular imaging and tracking at the push of a button!
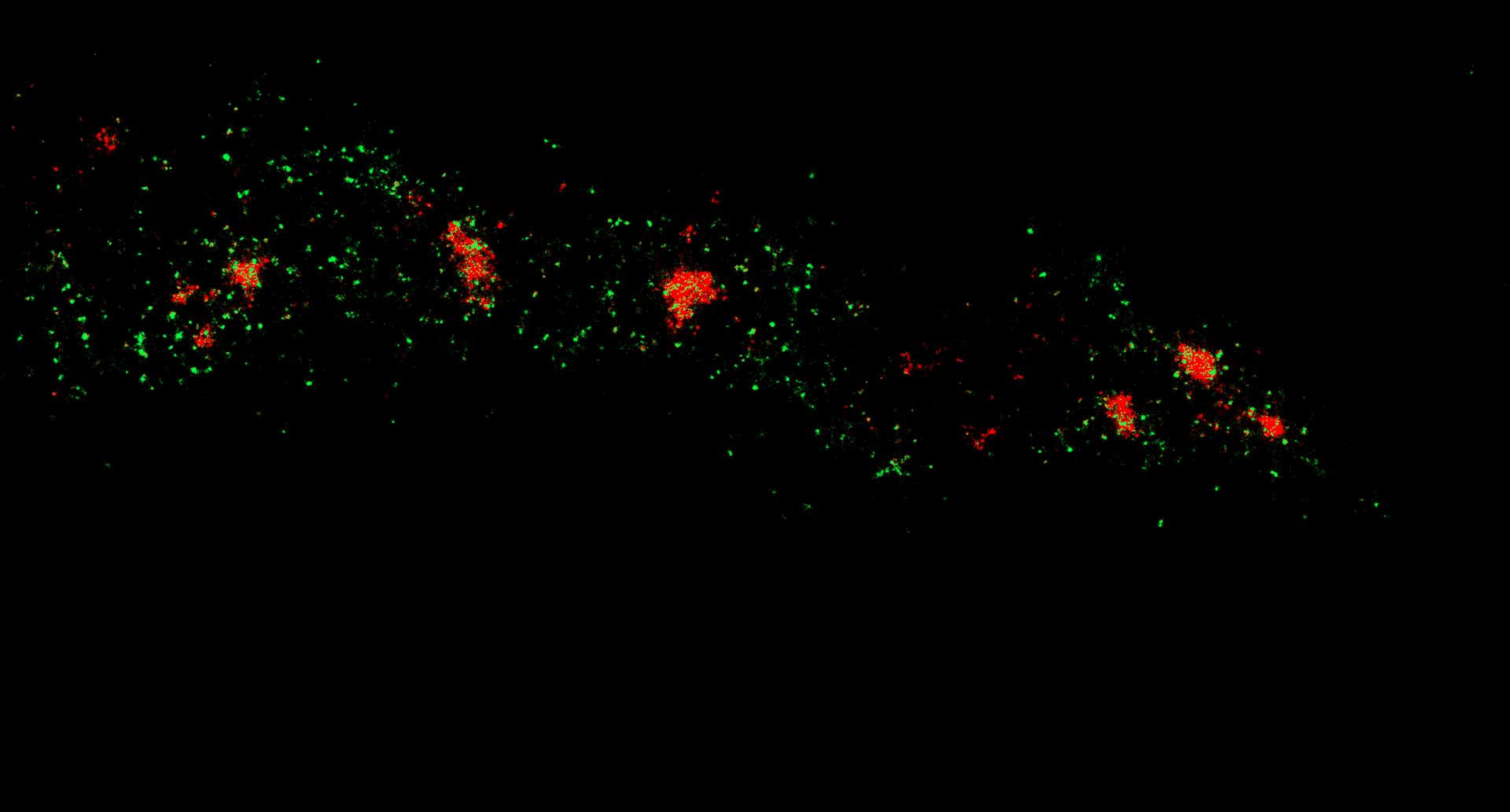
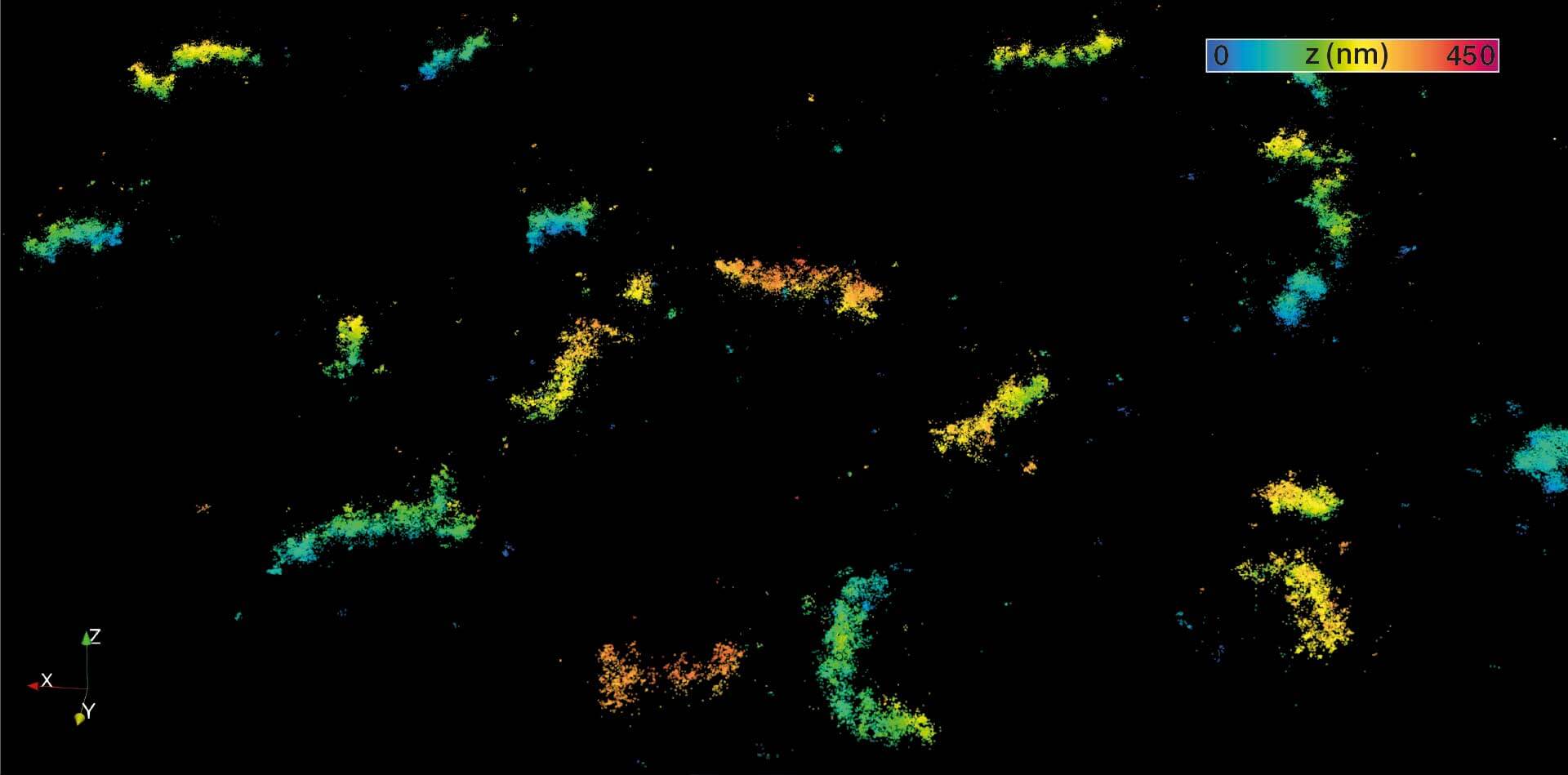
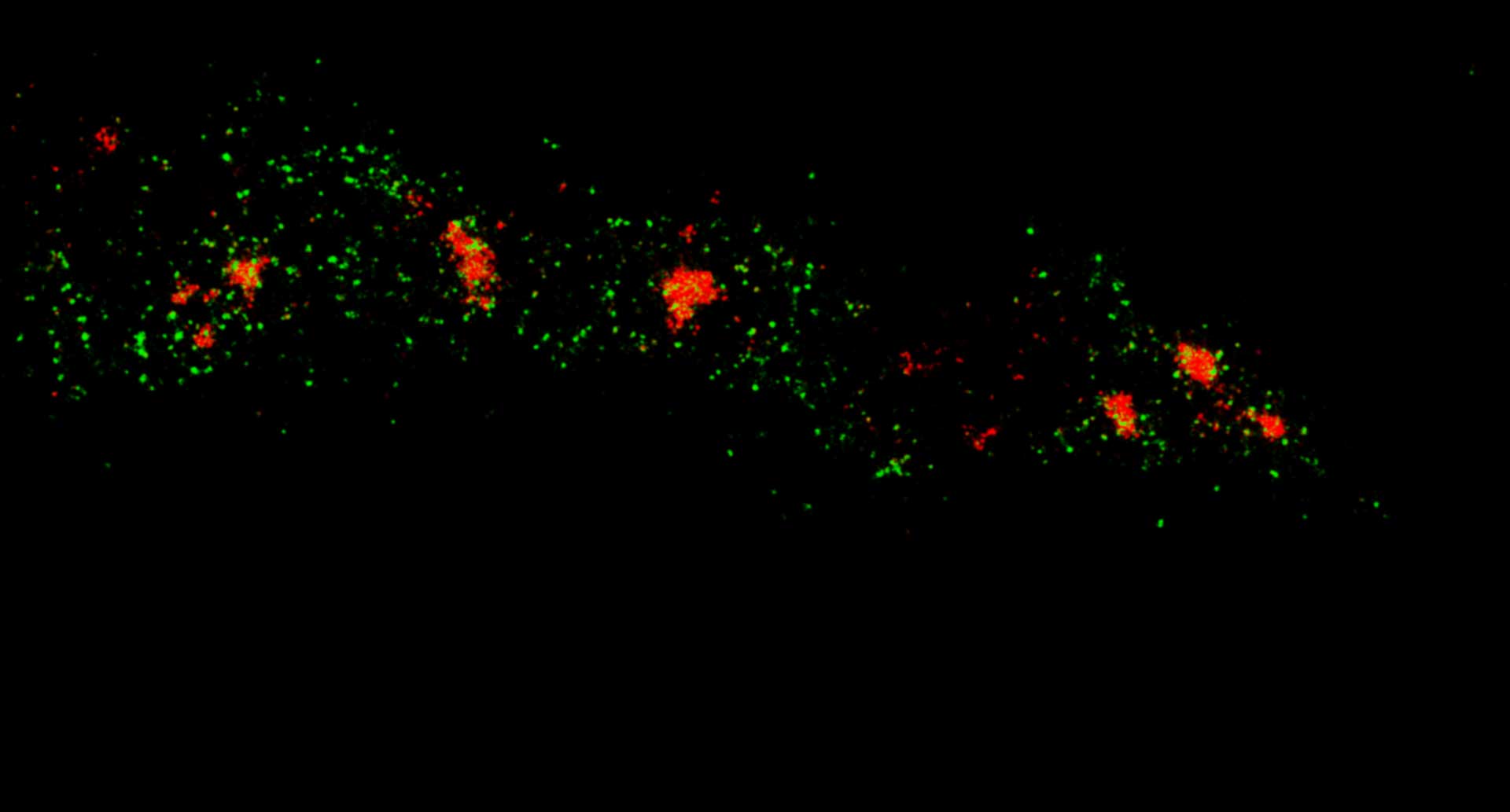
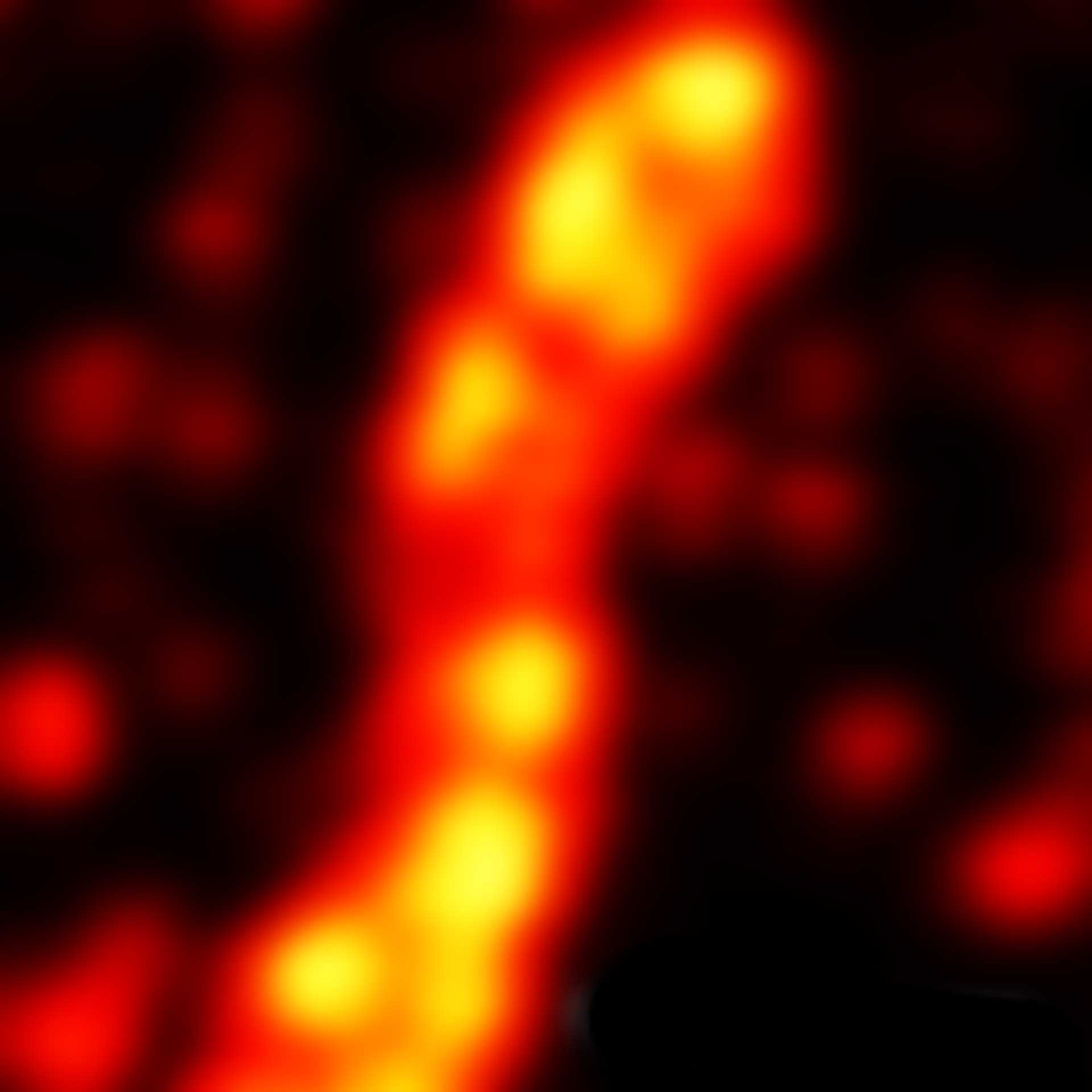
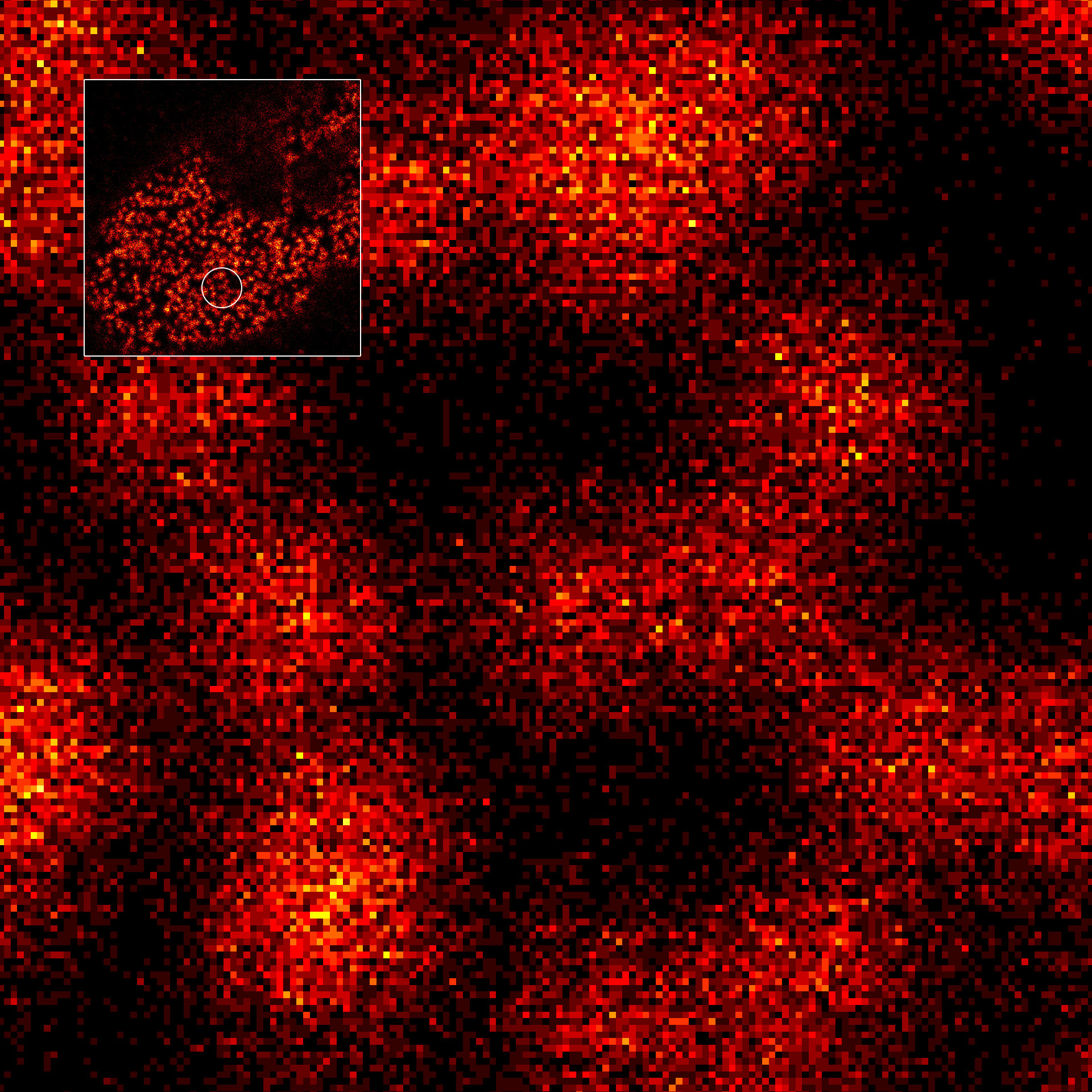
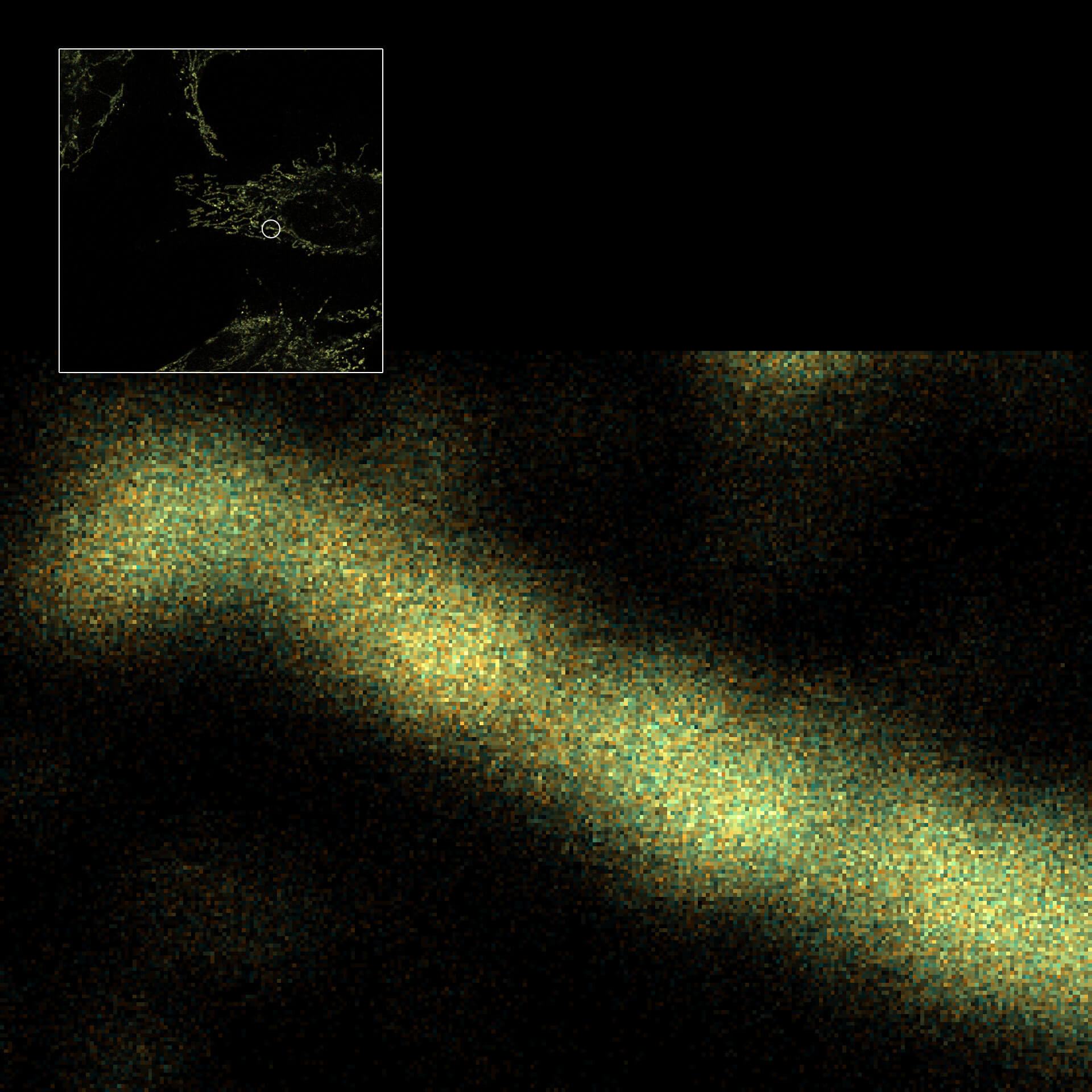
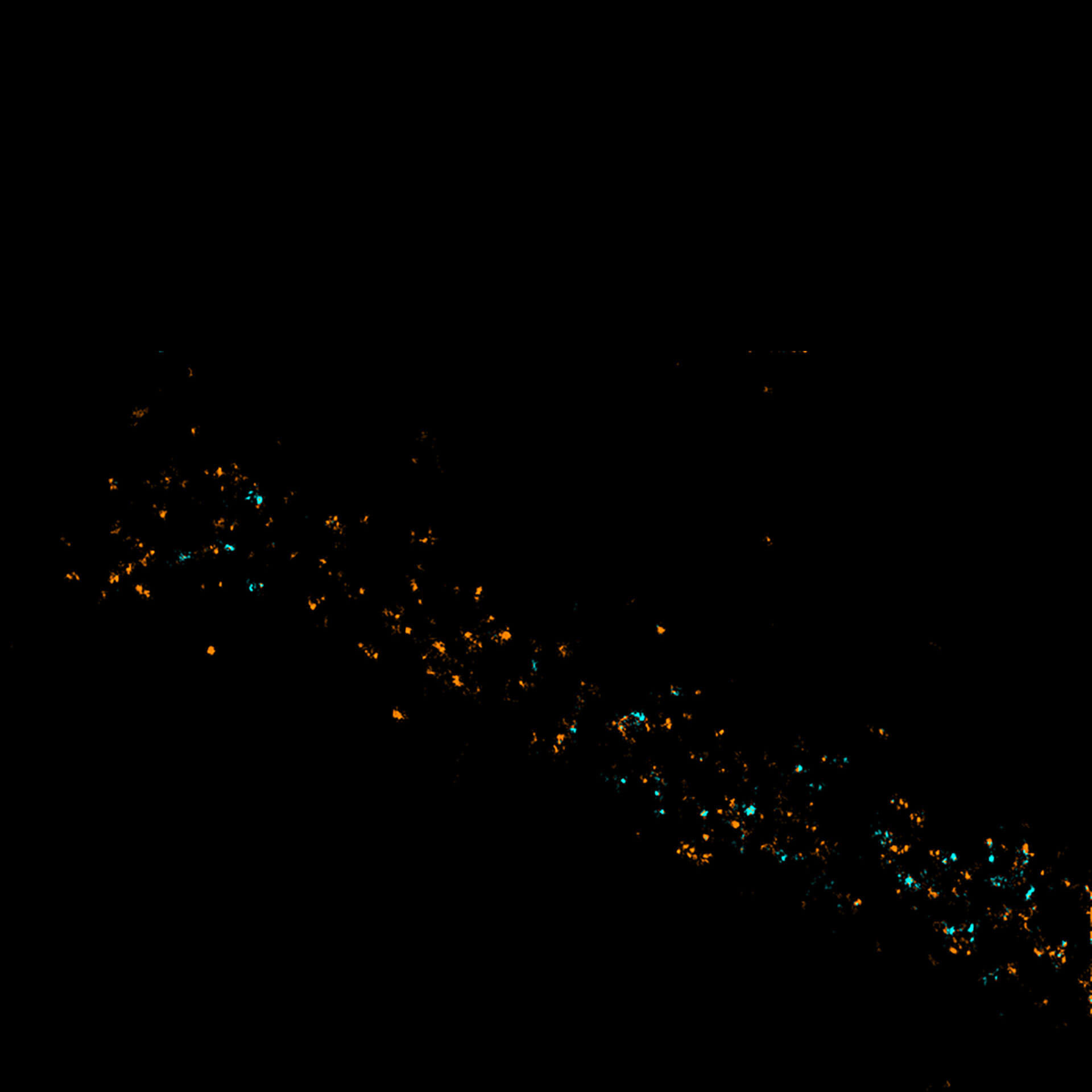
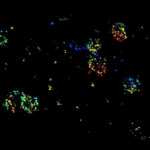
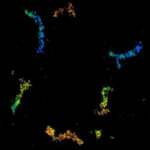
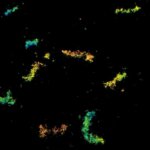
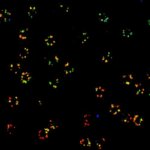
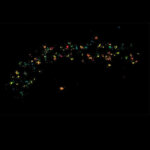
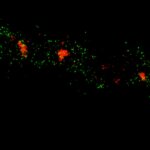
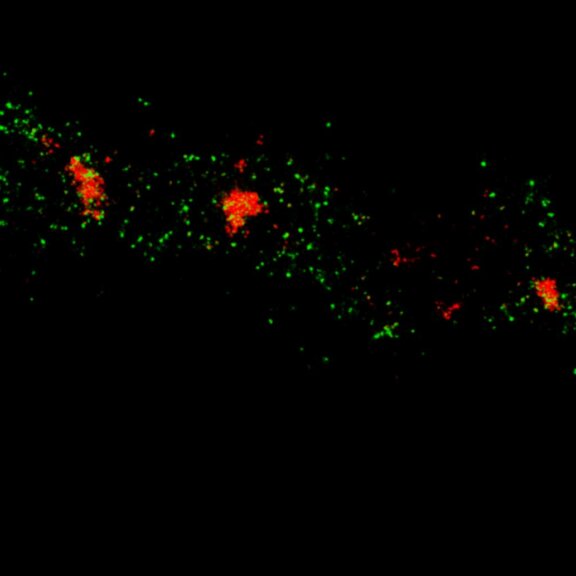
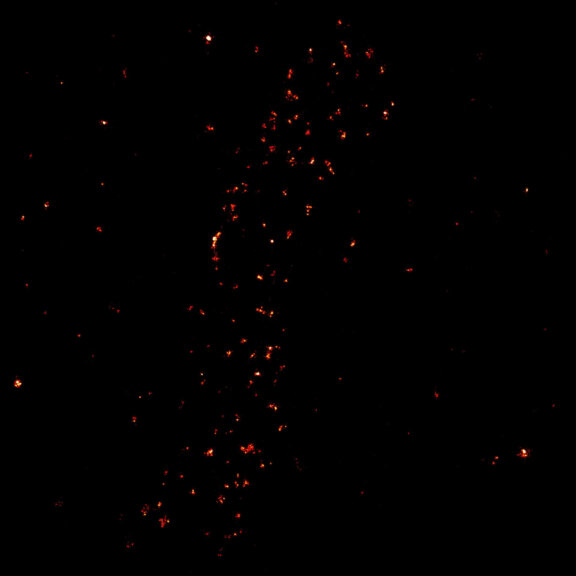
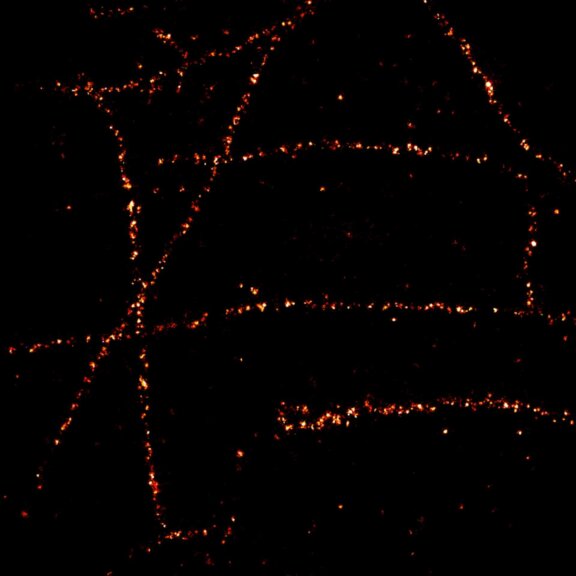
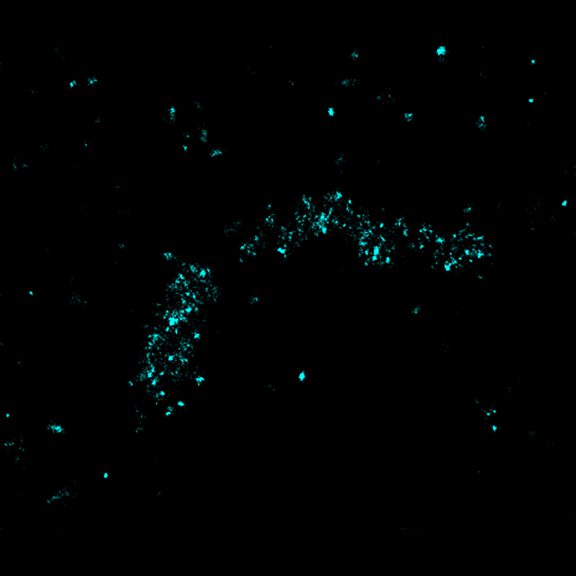
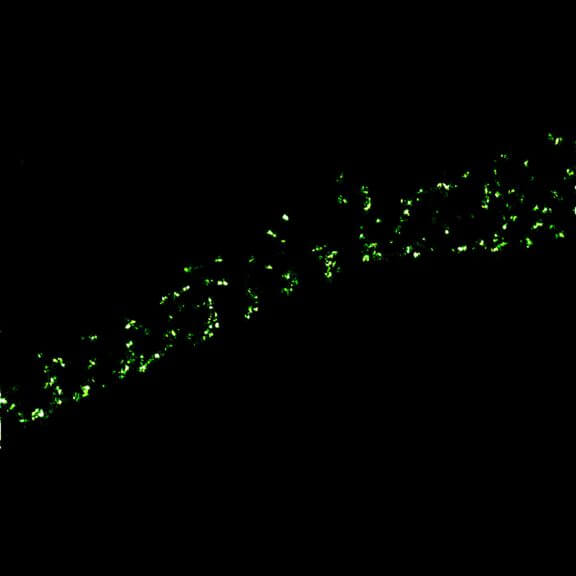
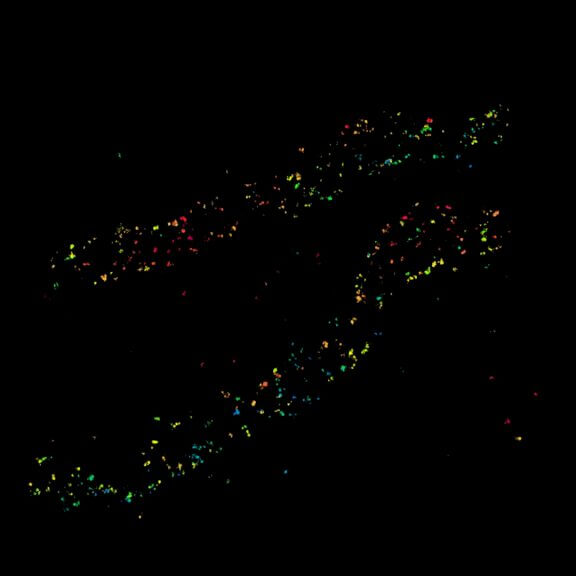
Two-color confocal and MINFLUX images of Tom20 (green) and mitochondrial DNA (red) stained with sCy5 and CF680 in mammalian cells using indirect immunolabeling. The two fluorophores were distinguished by ratiometric detection strategy. Note the dissimilar labeling density of the two imaged structures.
Maximize resolution
with minimal emission
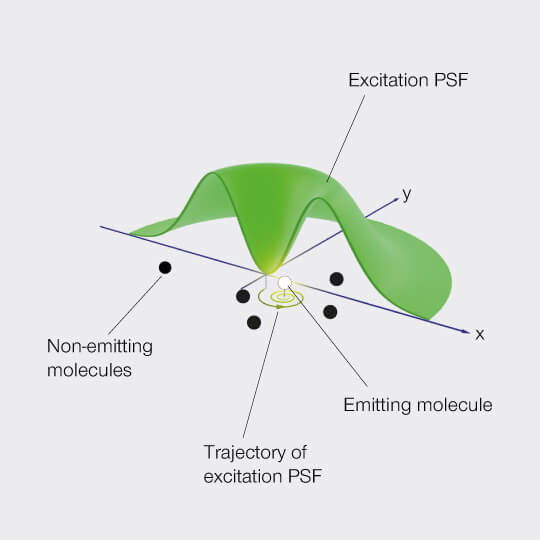
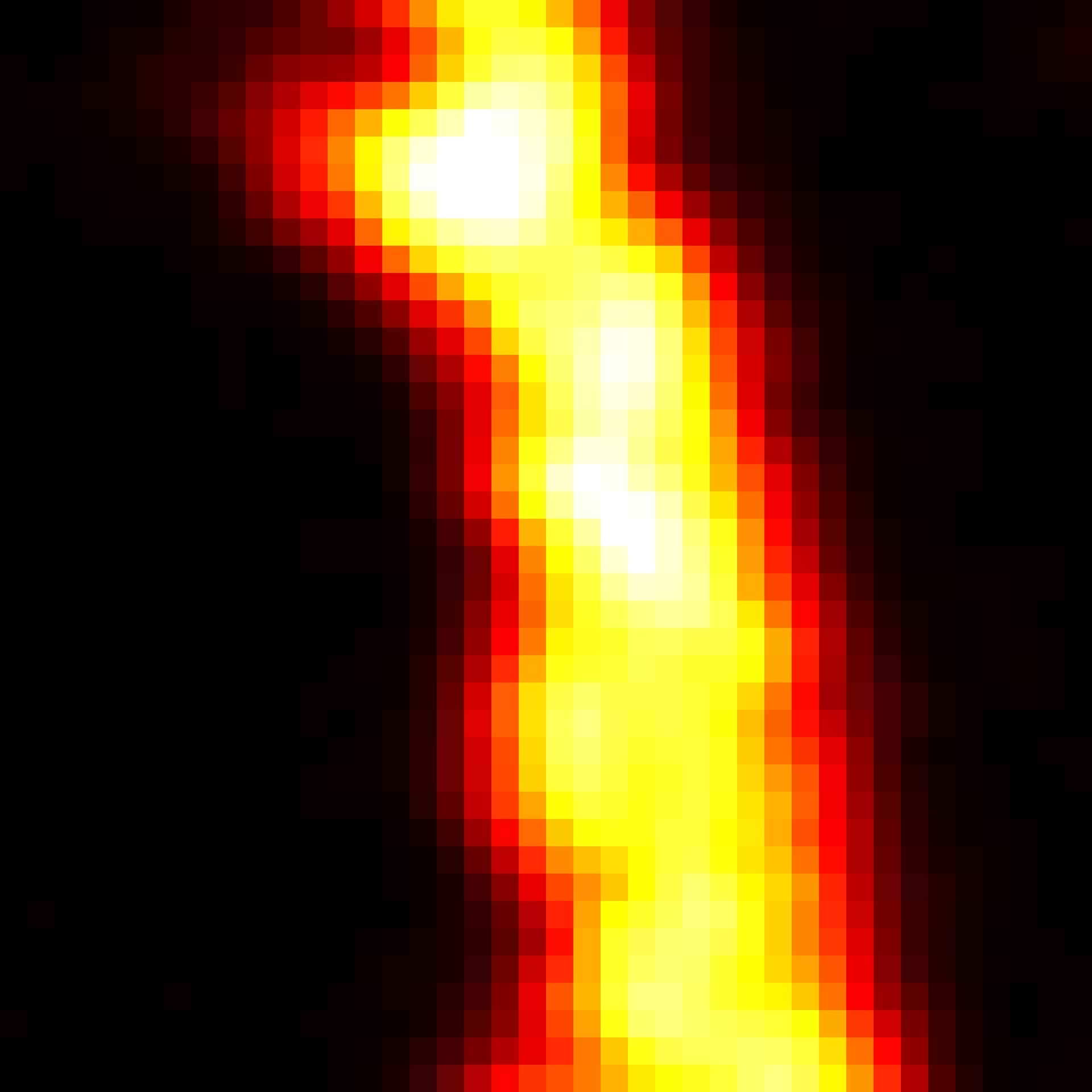
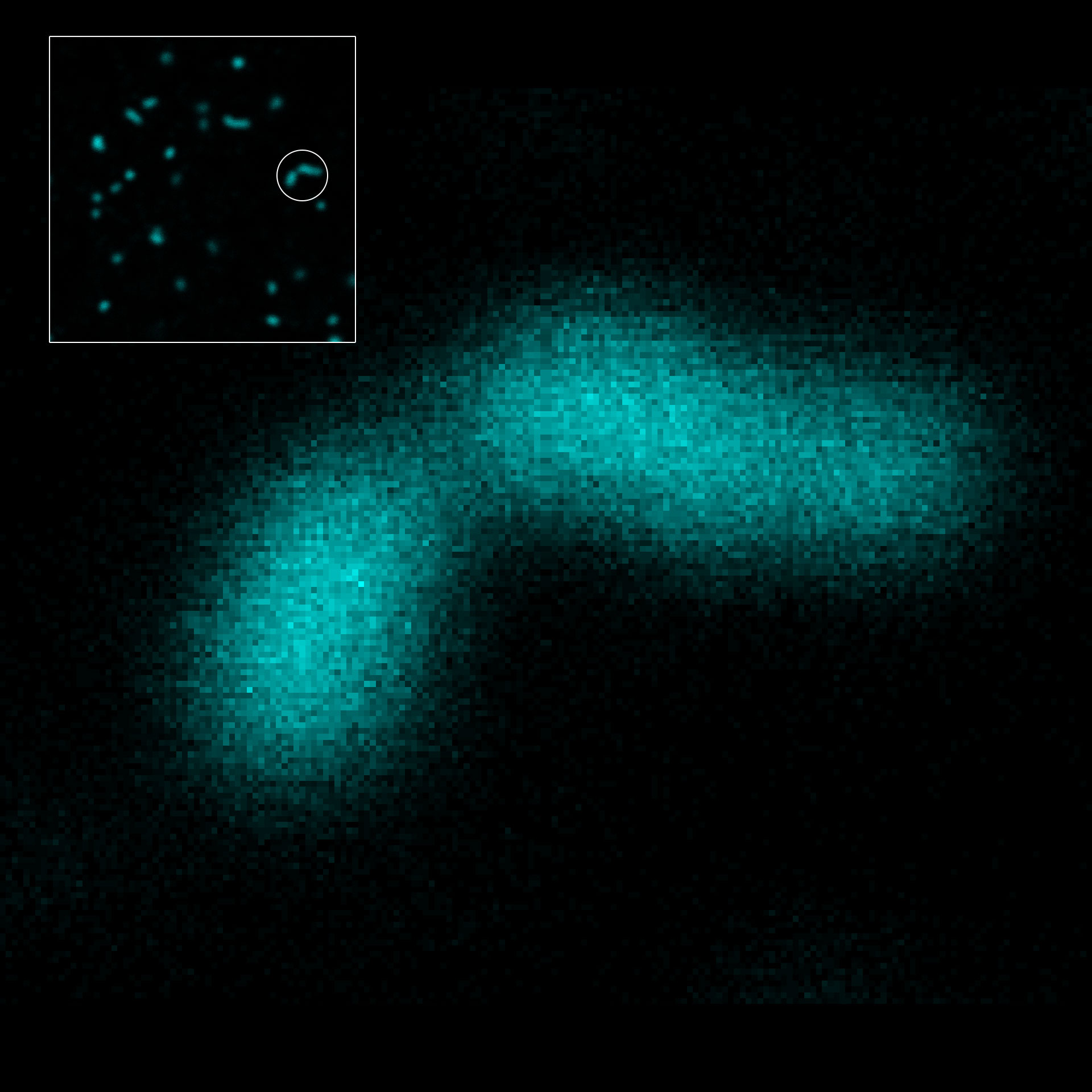
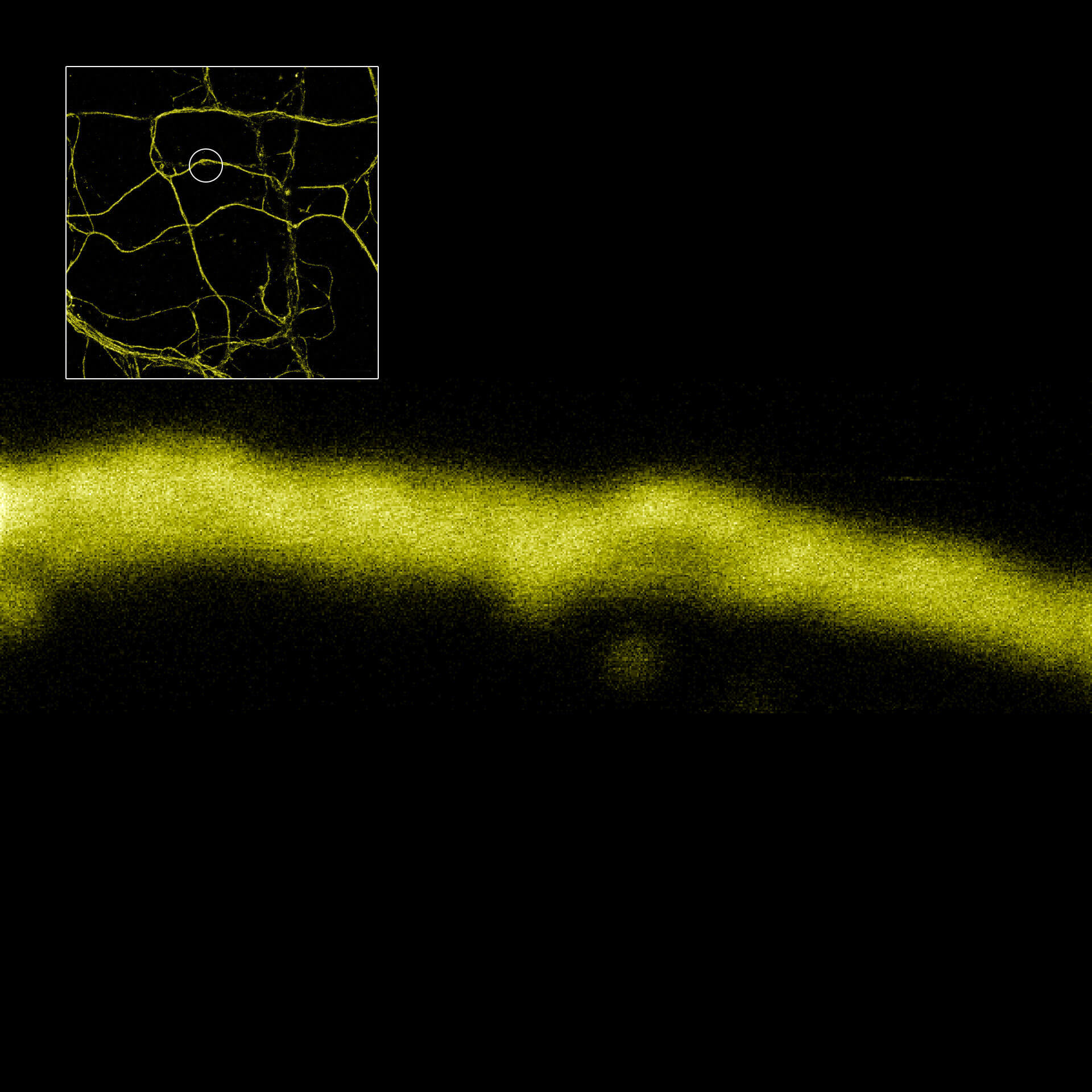
MINFLUX defines an entirely new class of superresolution methods that uses the best of STED microscopy and the single molecule localization family: 1) Emitters are activated one-at-a-time to obtain the best molecule separation possible, 2) Localization is performed with a light distribution for fluorescence excitation that has a central intensity zero instead of a maximum. This fundamentally reduces the number of emitted photons required for ultimate localization precision. The central intensity-zero of the excitation beam searches for the emitting molecule by performing a clever sequence of sub-nm sized probing steps. The closer the intensity-zero is to the molecule, the lower the resulting fluorescence. By optimizing for low emission rates, the MINFLUX microscope zooms in on the molecule, concomitantly increasing the precision with which the molecular position is revealed.
MINimizing fluorescence FLUXes by matching the dark center of the excitation beam with the molecule‘s position localizes molecules reliably and with 1-3 nanometer precision in 3D! By relying on emission minimization rather than maximization, MINFLUX localization is: i) inherently fast, ii) does not discard weakly emitting molecules, iii) minimizes bleaching, and iv) is less drift dependent. Inaccuracies due to unknown molecular orientations and tumbling that severely compromise camera-based spot-centroid localization are ruled out.
Standard body
work as usual
The scientists and developers at abberior understand the importance of smooth and simple operation when performing biological research. That’s why our MINFLUX system is built around a standard microscope body that provides a variety of options ranging from widefield fluorescence, DIC, phase contrast over confocal and STED, all the way up to MINFLUX. To maximize extendability, we built our MINFLUX system with reliable and time-tested elements assembled on a robust optical breadboard. We use rock-solid opto-mechanical building blocks that have been proven to function seamlessly in hundreds of abberior microscopes all around the world.
Moreover, in line with our design philosophy for top-of-the-line instruments, our MINFLUX systems are future-proof: they are designed to allow adaptations with the latest technologies available: performance in perpetuity.
Almost no drift
solid as a rock
When performing experiments with nanometer resolution, miniscule sample drifts and movements can compromise performance. That’s why our MINFLUX comes with active sub-nanometer stabilization technology. When MINFLUX imaging is performed, a fully automated stabilization system based on laser-illuminated fiducial markers keeps the sample perfectly still, with residual fluctuations < 1 nm in 3D.
- more than 100 kHz line frequency: scan 100 times faster than any other method
- 1 – 3 nm localization precision in 3D: resolve 100 times better than confocal microscopy
- 100 µs time per localization: track fluorophores 100 times faster than with a camera